Amino acids contain both an amino (-NH2) and a carboxylic (-COOH) functional group.
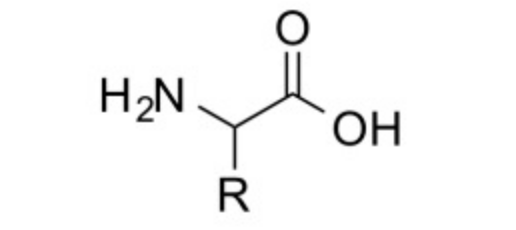 (Generic amino acid structure)
(Generic amino acid structure)
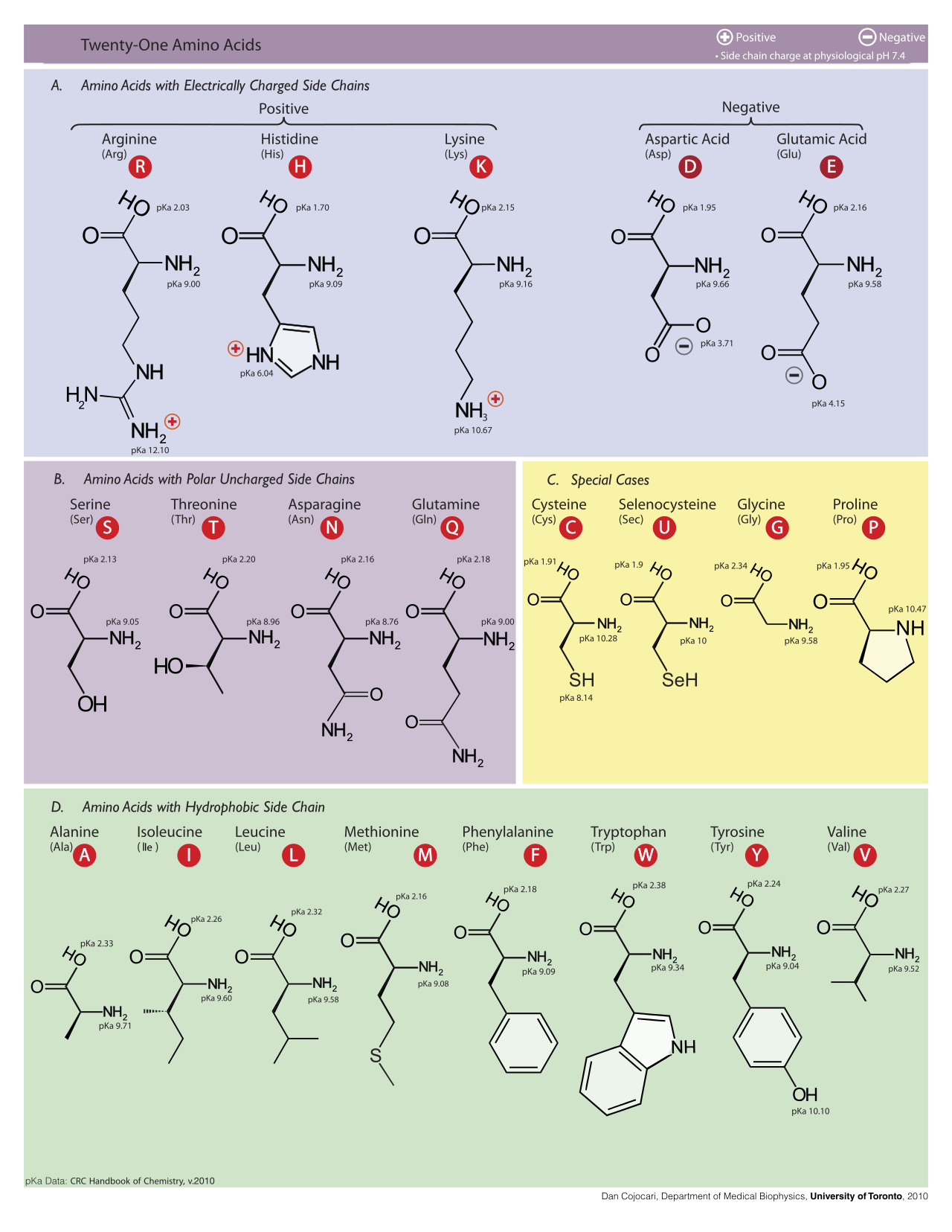 (Molecular structures of the 21 proteinogenic amino acids, Dan Cojocari, 2010. Wikipedia link)
(Molecular structures of the 21 proteinogenic amino acids, Dan Cojocari, 2010. Wikipedia link)
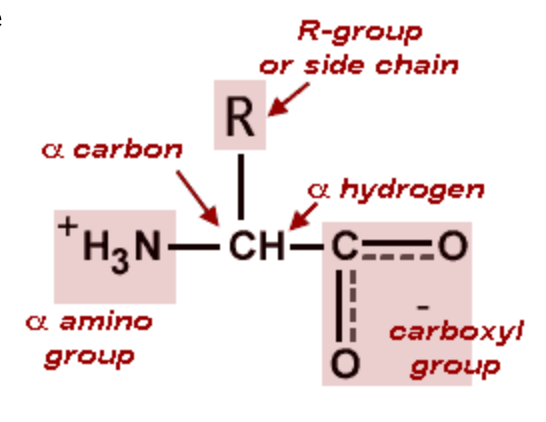
The carbon atom next to the carboxyl group is called the alpha-carbon. Amino acids containing an amino group bonded direclty to the alpha-carbon are referred to as alpha-amino acids.
pKa = acid dissociation constant. It indicates whether an acid is a strong acid or a weak acid. The lower the pKa, the more easily it gives up its proton. The higher the pKa, the more tightly the proton is held. Strong acids have pKa of less than zero.
At physiological pH (pH 7), most aas have a COO- group and a NH3+ group and are therefore zwitterions: -NH3+-CHR-COO-. This form predominates at pH values between the two pKas (the pKa of the amino group and the pKa of the carboxyl group). The so-called “neutral” forms -NH2-CHR-COOH are not present to any measurable degree at neutral pH. At strongly acidic conditions (pH < 3), the carboxylate group becomes protonated (-COOH), resulting in -NH3+-CHR-COOH. At strongly basic conditions (pH > 10) the ammonio group is deprotonated to give -NH2-CHR-COO-. The acidic amino acids have an additional COO- group at pH 7.
- Alanine
- Arginine
- Asparagine
- Aspartic acid
- Cysteine
- Glutamine
- Glutamic acid
- Glycine
- Histidine - essential
- Isoleucine - essential
- Leucine - essential
- Lysine - essential
- Methionine - essential
- Phenylalanine - essential
- Proline
- (Pyrrolysine)
- (Selenocysteine)
- Serine
- Threonine - essential
- Trypotphane - essential
- Tyrosine
- Valine - essential
Chemistry of amino acids
Acidic amino acids
- Misleading name, since those aas can accept a proton (and are therefore Bronsted bases).
- Aspartic acid and Glutamic acid
- Polar and negatively charged at physiological pH.
- Have two carboxyl groups.
- The corresponding amides Asparagine and Glutamine are polar and uncharged.
- Both acidic aas and the amides are very hydrophilic.
- Tyrosine and Cysteine, which act primarily as acids at neutral pH, are usually forgotten in this classification.
Aliphatic amino acids
- From least to most hydrophobic: Glycine, Alanine, Valine, Leucine, Isoleucine. Hydrophobicity increases with the number of C atoms in the hydrocarbon chain.
- Non-polar and hydrophobic.
- Prefer to remain inside protein molecules. Glycine and Alanine are ambivalent (i.e. can be inside or outside).
Aromatic amino acids
- Relatively non-polar.
- Absorb UV light. Tryptophane absorbs the strongest, at 280 nm.
- From least to most hydrophobic: Tyrosine, Trypotphane, Phenylalanine.
Basic amino acids
- Polar and positively-charged (-NH3+) at neutral pH.
- Very hydrophilic.
- Arginine, Lysine, Histidine.
- Almost always in contact with the solven (i.e. on outside of protein).
- Misleading classification, since Histidine acts as both Bronsted base and acid, Lysine acts primarily as Bronsted acid (i.e. accepts a proton), and Arginine has a fixed positive charge.
Cyclic amino acids
- Proline
- Non-polar
Hydroxyl amino acids
Sulfur-containing amino acids
- Cysteine, Methionine
- Non-polar, hydrophobic.
Interactions
Water-soluble proteins tend to have their hydrophobic resides buried on the inside, whereas hydrophilic residues are exposed to the outside. Integral membrane proteins have exposed hydrophobic amino acids that anchor them in the lipid bilayer. Proteins that have to bind to positely charged molecules have surfaces rich in negatively charged amino acids, and the other way round (e.g. low-complexity regions of nucleic acid-binding proteins contain a lot of lysine and arginine).