- Heterofermentative (only lactic acid) and homofermentative (lactic acid + ethanol + CO2)
- anaerob, but aero-tolerant
- Lactobacillus, Leuconostoc, Pedicococcus, Lactococcus, Streptococcus, Carnobacterium, Enterococcus, Oenococcus, Tetragenococcus, Vagococcus, Weisella
- They have limited biosynthetic ability, having evolved in environments that are rich in amino acids, vitamins, purines and pyrimidines, so they must be cultivated in complex media that fulfill all their nutritional requirements. Limited capacity to synthesize amino acids using inorganic nitrogen sources. They are therefore dependent on preformed amino acids being present in the growth medium as a source of nitrogen.
- Contribute to the taste and texture of fermented products and inhibit food spoilage bacteria by producing growth-inhibiting substances and large amounts of lactic acid.
- Ability to degrade different carbohydrates and related compounds (but depending on species and strain!).
- Spoilage organisms among LAB typically grow slowly with lactic acid as a carbon source.
Fermentation pathways
Presence or absence of the key cleavage enzymes of the glycolysis pathway (fructose 1,6-diphosphate) and the pentose phosphate pathway.
Homofermentative
Glycolysis pathway. Some lactobacilli and most species of enterococci, _lactococci, pediococci, streptococci, tetragenococci, and vagococci.
- Under excess gluose and limited oxygen: one mole of glucose yields two moles of pyruvate (through glycolysis pathway). Oxidation of NADH to NAD, while puryvate gets reduced to lactate. The process yields two molecules ATP per glucose consumed.
- Free glucose is transported into the cell and phosphorylated by glucose-6-phosphate by an ATP-dependent hexose kinase. Other sugars (mannose, fructose) enter the major pathway at the level of glucose-6-phosphate or fructore-6-phosphate after isomerization or phosphorylation, or both.
- Alternatively, some species use the phosphoenolpyrucate (PEP) sugar phosphtransferase system (PTS); either for galactose only or for all sugars.
Heterofermentative
Pentose phosphate pathway. Leuconostocs, some lactobacilli, oenococci, and weissella species.
- One mole glucose ⇒ glucose-6-phosphate ⇒ dehydrogenated to 6-phosphogluconate ⇒ decarboxylated to yield one CO2.
- Pentose-5-phosphate is cleaved into one molecule of glyceraldehyde phosphate (GAP) and one molecule of acetyl phosphate ⇒ GAP is further metabolized into lactate; acetyl phosphate is reduced to ethanol via acetyl-CoA and acetaldehyade intermediates.
Proteolytic system
Since the quantities of free amino acids present in their environment are not sufficient to support the growth of bacteria to a high cell density, they require a proteolytic system capable of hydrolyzing peptides and proteins in order to obtain essential amino acids. All dairy lactococci used for acidification of milk (e.g., in cheese manufacture) have proteolytic activity. The lactococcal proteolytic system consists of enzymes outside the cytoplasmic membrane, transport systems, and intracellular peptidases. The proteolytic activity of LAB contributes additionally to the development of the flavor, aroma and texture of fermented products. For many varieties of cheeses, such as Swiss and Cheddar, desirable “flavor tones” are derived by proteolysis.
Lactobacillaceae
- 3 major subgroups, >250 species of which 84 are on the International Dairy Federation list of food cultures and around 20 are produced as starter culture or probiotic cultures.
- New taxonomy since 2020: reclassification into 26 genera: Lactobacillus, Pedicococcus, Paralactobacillus and 23 novel genera. 16S rRNA similarity allows robust assignment to the new genera.
 (Reclassified genera of lactobacillaceae and their translations. Source: Michael Gänzle talk for The Fermentation Association https://www.youtube.com/watch?v=ErQbie690po)
(Reclassified genera of lactobacillaceae and their translations. Source: Michael Gänzle talk for The Fermentation Association https://www.youtube.com/watch?v=ErQbie690po)
-
Convertion of old to new names: http://www.lactobacillus.uantwerpen.be/
-
More resistant to acidic conditions than other LAB. Can grow below pH 4 ⇒ often responsible for the final stages of lactic acid fermentation.
-
Very different characteristics among lactobacilli:
- Cocci or rods, may form chains, pairs, tetrads
- Respiring or non-respiring (facultative or stricly anaerobic)
- Homo- or heterofermentative
- Grow at 4ºC or at 45ºC, or at both temperatures
- Fermenting hexoses, pentoses or both
- Producing or consuming lactate
-
Safety: A few species can cause rare infections in critically ill patients, mostly in Weissella genus.
-
Antibiotic resistance: Lactobacillus, Amylolactobacillus and Holzapfelia are vancomycin sensitive, all other genera of Lactobacillaceae are vancomycin resistant. Some strains of intestinal Lactobacillaceae picked up tetracycline resistance.
-
Main product of fermentative metabolism is lactate, other products may be acetate, ethanol, CO2, formate or succinate.
Group 1
Homofermentative, produce lactic acid as major end product (>85%) from glucose. E.g. L. delbrueckii and L. acidophilus. Grow at 45ºC but not 15ºC.
Group 2
Homofermentative. Grow at 15ºC and show variable growth at 45ºC. E.g. L. casei and L. plantarum. Can produce more oxidized fermentations (e.g. acetate) when O2 is present.
Group 3
Heterofermentative. From glucose they produce lactic acid, ethanol and CO2. E.g. L. fermentum (now called Limosilactobacillus fermentum), L. brevis (Levilactobacillus brevi) and L. kefiri (Lentilactobacillus kefiri).
Lactobacillus
- Homofermentative
- Most species don’t ferment pentoses, but they have the ability to ferment a relatively broad spectrum of carbohydrates (e.g. mannitol), and even fructans, starch or glycogen. Metabolic focus on lactose explains the dominance in yogurt and cheese fermentations (esp. of L. delbrueckii), as well as the presence in the intestines of suckling pigs.
Lacticaseibillus
- Homofermentative
- Some species ferment pentoses via the phosphoketolase pathway.
- Several species of it are used as dairy starter cultures and probiotics.
Different species of Lactobacillaceae
Lactobacillus delbrueckii subsp. bulgaricus
Previously L. bulgaricus. Ferments glucose to D(-)-lactic acid and also metabolises fructose, mannose and lactose, but not sucrose. Important starter culture for yogurt and cheese. Type strain: ATCC 11842 (DSM 20081). Genome sequence accession number: JQAV00000000.
Lactobacillus delbrueckii subsp. delbrueckii
Ferments glucose to D(-)-lactic acid and also metabolises sucrose, fructose and mannose, but not lactose. Isolated from vegetables and fermented grains. Type strain: ATCC 9649 (DSM 20074). Genome sequence accession number: AY773949.
Lactobacillus delbrueckii subsp. lactis
Includes strains previously designated as L. lactis and L. leichmannii. Ferments glucose do D(-)-lactic acid and also metabolises sucrose, fructose, mannose, maltose and trehalose. Isolated from milk, cheese and grain mash. Type strain: ATCC 12315 (DSM 20072). Genome sequence accession number: AZDE00000000.
Lactobacillus acidophilus
Produces DL-lactic acid from cellobiose, galactose, lactose, maltose, mannose, sucrose and trehalose. Specific strains have been used as probiotics. Isolated from intestinal tract of humans and animals, human mouth, vagina, sourdough and wine. Type strain: ATCC 4356 (DSM 20079). Genome sequence accession number: AZCS00000000.
Lactobacillus helveticus
Produces DL-lactic acid from glucose, galactose, lactose, mannose, and trehaolse, but not cellobiose, mannitol, raffinose and sucrose. Isolated from sour milk, cheese (particularly Emmental and Gruyère cheeses). Type strain: ATCC 15009 (DSM 20075). Genome sequence accession number: AZEK00000000.
Streptococcus thermophilus
Streptococcus thermophilus is an alpha-hemolytic species of the viridans group. The bacterium is found in milk and milk products. It is not a probiotic (it does not survive the stomach) and generally is used in the production of yogurt and the manufacture of several types of cheese, especially Italian and Swiss cheeses. The organism is a moderate thermophile with an optimal growth rate at 45 °C. Although S. thermophilus is closely related to other pathogenic streptococci (such as S. pneumoniae and S. pyogenes), S. thermophilus is classified as a non-pathogenic species. It is closely related to S. salivarius in the oral cavity.
Lactococcus lactis
Lactococcus is a genus of of LAB with five major species formerly classified as Group N streptococci. The type species for the genus is L. lactis, which has two subspecies, lactis and cremoris. Lactococci differ from other lactic acid bacteria by their pH, salt and temperature tolerances for growth.
Lactococcus lactis is critical for manufacturing cheeses such as Cheddar, cottage cheese, cream cheese, Camembert, Roquefort and Brie, as well as other dairy products like cultured butter, buttermilk, sour cream and kefir. The bacterium can be used in single strain starter cultures, or in mixed strain cultures with other lactic acid bacteria such as Lactobacillus and Streptococcus.
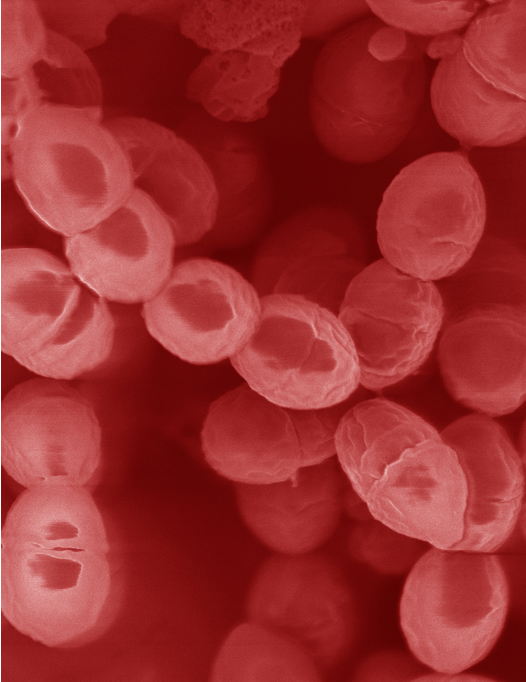 (Lactococcus lactis. UW Department of Bacteriology strain LcL325UW. Magnification 20000X. Scanning electron micrograph by Joseph A. Heintz, University of Wisconsin-Madison. From: https://textbookofbacteriology.net/lactics_5.html)
(Lactococcus lactis. UW Department of Bacteriology strain LcL325UW. Magnification 20000X. Scanning electron micrograph by Joseph A. Heintz, University of Wisconsin-Madison. From: https://textbookofbacteriology.net/lactics_5.html)
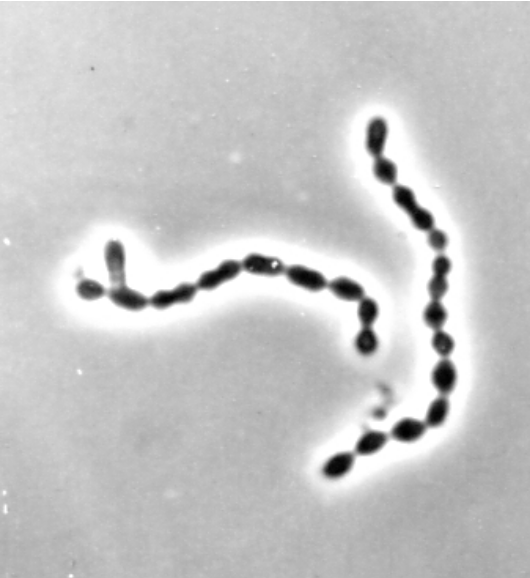 (Lactococcus lactis. Magnification 1500X. Phase micrograph courtesy of T.D. Brock, University of Wisconsin-Madison. From: https://textbookofbacteriology.net/lactics_5.html)
(Lactococcus lactis. Magnification 1500X. Phase micrograph courtesy of T.D. Brock, University of Wisconsin-Madison. From: https://textbookofbacteriology.net/lactics_5.html)
Lactococcus genome
Partly due to their industrial relevance, both Lactococcus lactis subspecies (lactis and cremoris) are widely used as generic LAB models for research. L. lactis ssp. cremoris, used in the production of hard cheeses, is represented by the laboratory strains LM0230 and MG1363. Currently, there are two L. lactis ssp. cremoris that have been sequenced for public release, one of which is L. lactic ssp. cremoris MG1363 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1855848/.
Similarly, L. lactis ssp. lactis is employed in soft cheese fermentations, with the workhorse strain IL1403, ubiquitous in LAB research laboratories. In 2001, the genome of strain IL1403 was sequenced leading to increased understanding of LAB genomics and related applications.
The genome sequence reveals 12 enzymes called aminotransferases, some of which are used to break down complex, branched, ring-shaped, and sulfur-containing amino acids. The molecules produced when the amino acids are degraded are very important for cheese flavor. Understanding which amino acids are broken down by which enzymes could give cheese makers greater control over flavor and fragrance of their cheeses.
Bifidobacterium
Bifidobacterium is not included in the traditional Lactic Acid Bacteria due to its genetic unrelatedness, but the bacterium has a habitat that overlaps with LAB, and it has a metabolism that produces lactic acid as a primary end-product of fermentation. Bifidobacteria are strictly anaerobic and are a prominant Gram-positve bacterium in the large intestine (colon). Bifidobacterium infantum is the predominant bacterium in the intestine of breast-fed infants because mother’s milk contains a specific growth factor that enriches for the growth of the bacterium.
Among 30 species, those recognized as probiotics include:
- B. adolescentis
- B. breve
- B. longum
- B. animalis
- B. infantis
- B. thermophilum
- B. bifidum
- B. lactis
Probiotics
Dairy fermentation
See my & Max Graze’s Dairy Ferments Airtable
Plant-based dairy fermentation notes
Vegetable fermentation
Bacteriocins and antibiotics from LAB
Nisin produced by Lactococcus lactis ssp. lactis has been studied extensively. It has a broad spectrum of activity against Gram-positive bacteria. The primary target is believed to be the cell membrane. Unlike some other antimicrobial peptides, nisin does not need a receptor for its interaction with the cell membrane; however, the presence of a membrane potential is required.
Nisin is a natural preservative present in cheese made with Lactococcus lactis ssp. lactis, but it is also used as a preservative in heat processed and low pH foods. Since nisin cannot be synthesized chemically, the nisin-producing Lactococcus lactis strains are used for its industrial synthesis.
The first established use of nisin was as a preservative in processed cheese products, but numerous other applications in preservation of foods and beverages have been identified. It is currently recognized as a safe food preservative in approximately 50 countries. Nisin has been used as a preservative in various pasteurized dairy products and canned vegetables, baked, high-moisture flour products, and pasteurized liquid eggs. There is interest in the use of nisin in natural cheese production. Considerable research has been carried out on the anti-listerial properties of nisin in foods and a number of applications have been proposed. Uses of nisin to control spoilage lactic acid bacteria have been identified in beer, wine, alcohol production, and high acid foods such as salad dressings. Production of highly purified nisin preparations has led to interest in the use of nisin for human ulcer therapy and mastitis control in cattle.
Although nisin is today the only bacteriocin that reached commercial status, approved worldwide as a natural food preservative, many other bacteriocins may soon reach similar status. Several lactobacilli having antibacterial activity against pathogens such as Listeria monocytogenes, Staphyloccus aureus, Salmonella newport and even E.coli.
Sources
- https://textbookofbacteriology.net/lactics.html
- Lactic Acid Bacteria: Food Safety and Human Health Applications. Ayivi et al., 2020. dairy
- The Fermentation Association seminar: The new taxonomy of Lactobacillus - Michael Gänzle & Ben Wolfe. 2021. https://www.youtube.com/watch?v=ErQbie690po
- 2020, Zheng et al. A taxonomic note on the genus Lactobacillus https://pubmed.ncbi.nlm.nih.gov/32293557/